For US Healthcare Professionals
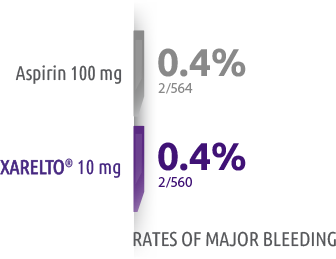
Similar rates of major bleeding
0.4% with XARELTO® versus 0.3% with aspirin
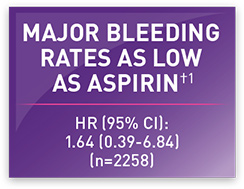
<1% major bleeding rates
*After 6 months initial treatment.
†Major bleeding for XARELTO® was 0.4% (5/1127) versus 0.3% (3/1131) for aspirin and was defined as overt bleeding that was associated with a decrease in the hemoglobin level of ≥2 g/dL, led to transfusion of ≥2 units of red cells, occurred in a critical site, or contributed to death.